Request an Action
The Request an Action section of the Protocol Actions page displays subsections for each available action. Available actions differ depending on factors such as previously-performed actions, pending action requests, the role of the logged-in user, and document status.
The standard initial actions available when a Researcher is working with a new Protocol document (with a status of Pending/In Progress) are:
• Submit for Review
• Delete Protocol, Amendment, or Renewal
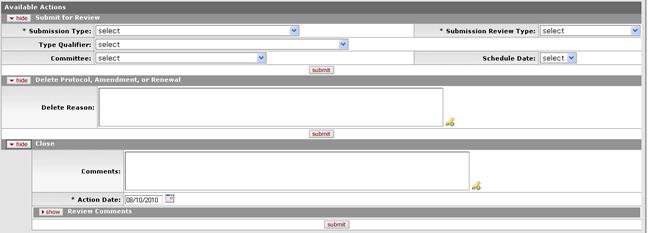
Figure 669 Protocol Document, Protocol Actions Page, Request An Action Section, Initial Available Actions Sections Layout
Select an Available Action link to navigate to subtopic instructions:
Subsequent Available Actions
The list of Available Actions changes dynamically based on the role of the user and the current state of the Protocol document. For example, the Researcher will see a different set of actions than an IRB Administrator; and before the Protocol document is approved, there will be a different set of actions than after it is approved.
|
|
“Follow-up actions” appear in the Request an Action section after certain actions are performed. Only those actions which can logically be performed given the new state of the Protocol document dynamically appear in the refreshed list of available actions. The logged-in user’s role and associated permissions also dictate the actions that are available. |
Depending on your role, previously-requested actions and the current document status, additional actions that can be requested appear as subsections with show/hide buttons to control their display, as shown in the following example:
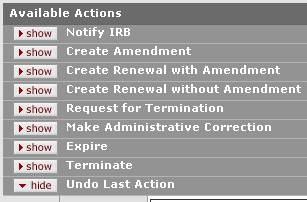
Figure 670 Protocol Document, Protocol Actions Page, Request an Action Section, Subsequent Available Actions Example
How-To Quick Reference: The following table identifies various Available Actions that are possible and briefly summarizes and describes how to execute the action using the entry, selection, and action tools provided in each corresponding subsection.
Table 290 Protocol Document, Protocol Actions Page, Request an Action Section, Subsequent Available Actions Descriptions (Alphabetical)
|
Available Actions Subsection |
Summarized Usage Description |
|
Approve Action |
IRB Administrator Only. After recording a vote on a motion to approve, the IRB Administrator records the decision to grant approval, enters the approval date and comments. Expiration date will be generated based on approval date, but can be modified. IRB Determinations can be recorded. |
|
Assign Reviewers |
IRB Administrator Only. Click the desired checkbox for each reviewer you want to select, choose either primary, secondary, or tertiary for each, then click the submit button. |
|
Assign to Agenda |
IRB Administrator Only. Click the Assign this protocol to an agenda checkbox, type to enter comments, select an Action Date as desired; optionally add Review comments, then click the submit button. |
|
Assign to Committee and Schedule |
IRB Administrator Only. Select a Committee and Schedule Date from the dropdown lists, then click the submit button. |
|
Close |
IRB Administrator Only. Enter comments, review comments and an effective date. |
|
Close Enrollment |
IRB Administrator Only. This is the IRB Administrator’s reaction to the Researcher’s request to close enrollment of study participants by the PI and it changes the status of the document to Active – Closed for Enrollment. This action can also be taken by an IRB Administrator without a request from the Researcher. |
|
Create Amendment |
Type to enter text in Summary; select appropriate checkbox(es) for the portion of the Protocol document in need of amendment in Amend, then click the create button. (Note that the Add / Modify Attachments and Notes box must be selected in order to upload revised documents.) A new Protocol document with the A00X suffix is created, and you are taken to the Questionnaire tab to complete the Amendment Questionnaire. |
|
Create Renewal with Amendment |
Type to enter text in Summary; select appropriate checkbox(es) for the portion of the Protocol document in need of amendment (if applicable) in Amend, then click the create button. A new Protocol document with the R00X suffix is created, and you are taken to the Questionnaire tab to complete the Renewal and Amendment Questionnaires. Changes to the data elements and an extension of the expiration date will result upon approval. |
|
Data Analysis Only |
IRB Administrator Only. Response to Researcher request for Data Analysis Only, this action indicates that that the participant interaction portion of the study is complete and only data analysis remains to be completed. This action can also be taken by the IRB Administrator without a request from the Researcher. |
|
Defer Action |
IRB Administrator Only. Enter comments pertaining to the reason for deferral, specify an Action Date for the deferment action to become effective, optionally add Review Comments, then click the submit button. |
|
Delete Protocol, Amendment, or Renewal |
When the Protocol document has never been submitted, this allows you to delete it from the system. |
|
Expedited Approval |
IRB Administrator Only. IRB Administrator records decision to grant expedited approval. Enters the approval date and comments. Expiration date will be generated based on approval date, but can be modified. IRB Determinations can be recorded. |
|
Expire |
IRB Administrator Only. Enter comments that summarize the reason for the expiration, then select an action date for the expiration to become effective. |
|
Grant Exemption |
IRB Administrator Only. Enter comments that summarize the reason for the expiration, then select an action date for the expiration to become effective. |
|
Make Administrative Correction |
IRB Administrator Only. Type to enter comment text explaining the purpose for the correction, then click the edit button. Make corrections in the appropriate sections of the Protocol document, then click the save button to record changes. After all changes have been made and saved, click the close button. |
|
Manage Review Comments |
IRB Administrator Only. Enter review comments without performing any additional action on the protocol. |
|
Modify Submission Request |
IRB Administrator Only. Select different or additional categories for Exempt or Expedited protocols, change qualifiers for certain IRB Review not required submissions. Select different options for Submission Type, Submission Review Type, Billable flag, and Type Qualifier, and then click the submit button. |
|
Notify IRB |
Select a Submission Type and Submission Review Type (required), then click the create button. A new F00X protocol document is created, and you are taken to the Protocol tab. This is used by the Researcher to inform the IRB of events that require prompt reporting, such as adverse events or major protocol deviations, and noncompliance. |
|
Request for Data Analysis Only |
The Investigator submits a request to notify the Committee that the participant interaction portion of the study is complete, and only data analysis remains to be completed. A comment is included, and a Protocol Submission record is created. |
|
Request for Suspension |
Investigator submits a request to suspend the study. A reason for the suspension is required. Creates submission record. |
|
Request for Termination |
Investigator submits a request to terminate the study. A reason for the termination request is required, then click the submit button. Creates submission record. |
|
Request to Close |
Investigator submits a request to the IRB to close the study. A reason for the close request is required, then click the submit button. Creates submission record. |
|
Request to Close Enrollment |
Investigator submits a request to close enrollment of participants into the study. A comment is included. Creates protocol submission record. The request to close enrollment requires action by an IRB Administrator to close enrollment. |
|
Response Approval |
IRB Administrator Only. IRB Administrator records approval action resulting from a review of the Researcher’s response to a previously-reviewed protocol that required revisions. Enters the approval date, risk level, and comments. For Renewals, expiration date will be generated based on approval date but can be modified. Generates approval letter for review and to mark final. |
|
Suspend |
IRB Administrator Only. IRB Administrator marks protocol as suspended. Enter comments, reviewer comments, and action date. |
|
Suspend By DSMB |
IRB Administrator Only. Protocol is suspended by Data Safety Monitoring Board (DSMB). IRB Administrator enters comments, reviewer comments, and action date. |
|
Terminate |
IRB Administrator Only. Enter comments that summarize the reason for the termination, then select an action date for the termination to become effective. |
|
Undo Last Action |
Type to enter comments that summarize the purpose of undo-ing the last action, then click the submit button. |
|
Withdraw Protocol |
Type a textual reason for the withdrawal, then click the submit button to withdraw a Protocol application from review. The PI and any Correspondents are automatically notified. |
When Logged In As A Researcher
Contents
In this topic (select a Protocol Status link to navigate to subtopics):
- Unsubmitted Protocol – Status: Pending/In Progress
- Submitted Protocol – Status: Submitted To IRB
- Approved Protocol – Status: Active – Open to Enrollment

 Common Protocol Document Life Cycle
Stages By User Type/Status
Common Protocol Document Life Cycle
Stages By User Type/Status