Notify IRB
A Notify IRB action is used to submit prompt reports (e.g. adverse events, protocol deviations, etc.), noncompliance reports, audit reports, and any other ‘general information’ that doesn’t fall into the amendment or renewal category. A Notify IRB action can only be performed on protocols in active or exempt statuses.
Table 326 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Notify IRB Field Descriptions
|
Action attributes |
Description |
|
Who can perform action |
Principal Investigator, Co-PI Student/Fellow/Resident, Study Managers/Coordinator, and Aggregator. |
|
Protocol state prior to action |
Prior to the action being performed, the protocol must be in one of the following Statuses:
The Submission Status can be in any state.
|
|
Protocol state after action |
After the action is performed:
|
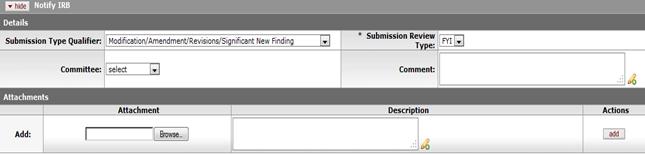
Figure 705 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Notify IRB Action Layout
Table 336 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions Subsection - Notify IRB Field Descriptions
|
Field |
Description |
|
Submission Type Qualifier |
Use
the drop-down
|
|
Submission Review Type |
The system auto-populates the only option, which is FYI |
|
Comment |
Optional. Enter any additional information necessary. Click within the text box to type or paste text as necessary to provide the appropriate information. Click the add note icon |
|
|
Click the create button to create the FYI submission. You are then able to upload documents on the Notes & Attachments tab but will be prohibited from making any additional changes to the Protocol. The system creates a new protocol document with the F00X suffix. Once the FYI is approved, its protocol attachments are merged with the original protocol in the History section, and the separate FYI item with the suffix of F00* ceases to exist.
It is possible to have more than one FYI pending at the same time. |

 to view/edit/paste text in a new browser window, then click the continue button to return to the text entry field in the document.
to view/edit/paste text in a new browser window, then click the continue button to return to the text entry field in the document.