Approve
The approve action allows the IRB Administrator to record a protocol approval following a full committee review.
This action only appears after the Record Committee Action has been performed on the protocol with a motion type of Approve. This action may be considered the final action on the lifecycle of an IRB protocol following which the research on human subjects can commence.
Table 293 Protocol Document, Protocol Actions Page, Request an Action Section, Approve Action - Action Attributes
|
Action attributes |
Description |
|
Who can perform action |
IRB Administrators are allowed to perform this action |
|
Protocol state prior to action |
Prior to the action, the protocol must be in the following state:
The Record Committee Decision must have been performed with a motion type selection of “Approve”. The protocol status must be Submitted to IRB. The submission status must be In agenda. |
|
Protocol state after action |
After the action is performed the protocol state changes as follows
The protocol status changes to Active - Open to Enrollment. The submission status changes to Approved. |
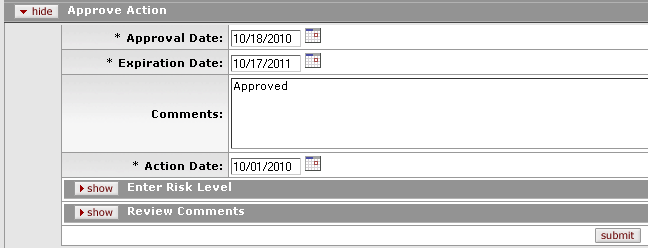
Figure 680 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Approve Action Layout
Table 294 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Approve Action Field Descriptions
|
Field |
Description |
|
Approval Date |
Specify the date you want the approval action to become
effective. By default, the field displays the current date. To
change it, click the calendar |
|
Expiration Date |
Specify the date in which the approval will
expire. By default, the field displays the current date. To
change it, click the calendar |
|
Comments |
To enter textual information to describe this action,
click within the text box (or press the tab |
|
Action Date |
Specify the date you want this action to become
effective. By default, the field displays the current date. To
change it, click the calendar |
|
Enter Risk Level | |
|
Add |
Displays sequential line item number for each table row in ascending, top-to-bottom order. Also serves as a line selection/addition row label. |
|
Risk Level |
Select one of the following options: • No greater than minimal risk. • Greater than minimal risk but potential for direct benefit for participant • Moderate Risk • Research involving greater than minimal risk, with no potential for benefit to participant, but likely to yield generalizable knowledge about the participant’s condition • High Risk Use the drop-down
|
|
Date Assigned |
By default, this displays the current date. To
change it, click the calendar |
|
Date Inactivated |
When the status is inactive, the date it became inactive is displayed. |
|
Status |
Display-only (for example, active). The current status of the risk level. |
|
Comments |
To enter textual information to describe the selection
of the risk level, click within the text box (or press the tab |
|
Add |
Click the add
|
|
Review Comments |
|

 key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information. Click the add note
key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information. Click the add note  icon to view/edit/paste text
in a new browser window, then click the continue button to return to the
text entry field in the document. After saved, click the green arrow
icon to view/edit/paste text
in a new browser window, then click the continue button to return to the
text entry field in the document. After saved, click the green arrow
 symbol to view full
text in a separate browser window.
symbol to view full
text in a separate browser window.

 button to add your selection to
as a row in the table below, which will become a numbered line item.
Click the delete
button to add your selection to
as a row in the table below, which will become a numbered line item.
Click the delete button to remove a previously-added line item row from the table.
button to remove a previously-added line item row from the table.