Create Renewal with Amendment
Currently, all renewals should be submitted as Renewals with Amendments, even if an Amendment is not included. This allows documents to be uploaded to KC IRB, such as a stamped consent.
Protocols are typically approved for a period of either one year or two years. If an investigator wants to renew a protocol, they need to submit a Renewal request. The Renewal with Amendment action allows the investigator to submit a renewal request and make changes to the research protocol at the same time. This must occur at least once per year for all protocols that are not federally funded or regulated and/or deemed to be greater than minimal risk, or once every two years for minimal risk studies that are not federally funded or regulated.
Table 307 Protocol Document, Protocol Actions Page, Request an Action Section, Create Renewal with Amendment – Action Attributes
|
Action attributes |
Description |
|
Who can perform action |
Principal Investigator, Co-PI Student/Fellow/Resident, Study Manager/Correspondent, and Aggregator can initiate this action. |
|
Protocol state prior to action |
Prior to the action being performed, the protocol must be in the following Status:
The Submission Status can be in any state.
|
|
Protocol state after action |
After the action is performed
|
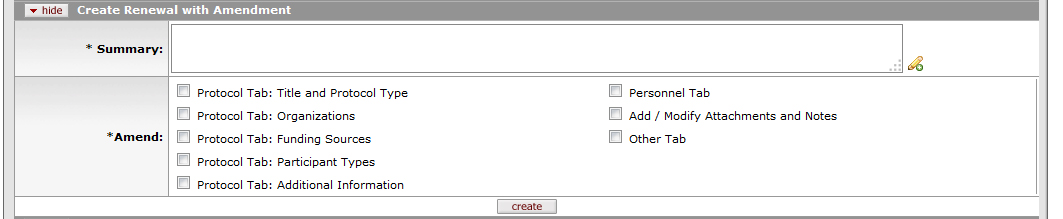
Figure 687 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions Subsection - Create Renewal with Amendment Layout
Table 308 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions Subsection - Create Renewal with Amendment Field Descriptions
|
Field |
Description |
|
Summary |
Enter a summary of the renewal and what is being amended, if anything; be as specific as possible for any amendment information. Click within the text box to type or paste text. Click the add note |
|
Amend |
Select one or more portions of the Protocol document, and/or Questionnaires if they are active for this study, that you intend to amend. A check mark appears within the check box to indicate the item is selected. Note that you must select the Add/Modify Attachments and Notes box in order to upload any revised documents. If there is already an outstanding amendment for the protocol, any portions being updated by it will not be available to modify for this submission.
If no changes are being made, still select at least one box in the Amend section, such as Add/Modify Attachments and Notes so stamped approved documents can be uploaded by HSO staff. |
|
|
Click the create button to initiate a new Renewal with Amendment. You will then be able to make changes to the portions selected under Amend.
The Questionnaire Tab will open, and three Renewal with Amendment Questionnaires will be listed under Submission Questionnaires. Questionnaires that have not been selected for editing, or that do not apply to this study, will be listed under Read-Only Questionnaires.
The system will create a Renewal document with the number of the protocol being renewed/amended followed by a suffix of R001 for the first renewal, R002 for the second renewal and so on. Once the Renewal is approved, the changes are merged with the original protocol, and the separate renewal document with the suffix of R00* ceases to exist.
It is possible to have more than one renewal or amendment outstanding at the same time, but to avoid conflicting information KC does not allow two renewals or amendments to update the same section of the protocol at the same time. Once any amendment to a protocol is approved, study information in all outstanding amendments to the same protocol will be updated automatically by the system to reflect the changes to the original protocol made by the approved amendment |
 icon to view/edit/paste text in a new browser window, then click the continue button to return to the text entry field in the document.
icon to view/edit/paste text in a new browser window, then click the continue button to return to the text entry field in the document. 