Submit for Review
The Submit for Review action allows the investigators to submit protocols, amendments, renewals and response to revision requests to the IRB. The system allows for investigators to select the review type for the protocol, as well as the Category of research for Exempt and Expedited submissions.
Table 362 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Submit for Review – Action Attributes
|
Action attributes |
Description |
|
Who can perform action |
This action can be initiated by investigators. |
|
Protocol state prior to action |
Prior to the action being performed, the protocol must be in the following Status:
The Submission Status can be in any state. |
|
Protocol state after action |
After the action is performed: The Submission Status changes to Pending
|
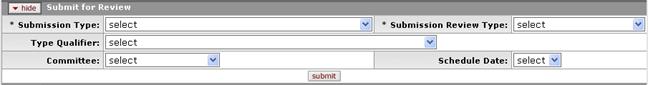
Figure 719 Protocol Document, Protocol Actions Page, Request An Action Section, Available Actions – Submit for Review Layout
Table 363 Protocol Document, Protocol Actions Page, Request An Action Section, Available Actions – Submit for Review Field Descriptions
|
Field |
Description |
|
Submit for Review | |
|
Submission Type |
The Submission Type will default to the appropriate type based on the type of submission you are creating:
|
|
Type Qualifier |
Only required for certain types of submissions. Use the drop-down menu by clicking the down arrow to display the list, and then click on an item in the list to select it.
When Submission Type = FYI, one of the following options must be selected:
When Protocol Type = Deferral and Submission Type = Initial Protocol Application, one of the following options must be selected:
When Protocol Type = Non Human Subjects Research and Submission Type = Initial Protocol Application, one of the following options must be selected:
|
|
Submission Review Type |
This field will default to the appropriate value based on the Protocol Type indicated on the Protocol tab. |
|
|
Click to submit the review request. Persons with designated protocol roles will receive the Action Request in their Action Lists. |

