IACUC Protocol
The Protocol document is an e-doc that works as an electronic protocol application form created by an investigator, which is submitted to the institution’s Institutional Animal Care and Use Committee (IACUC), and then reviewed by its committee members. The document will track required revisions, approval amendments, renewals and expiration. It is configurable by implementing institution to follow local institutional policies and practices in proposed research to be able to assess the risk/benefit ratio for the proposed research project.
|
In this topic: Preface |
Jump to page subtopics:
|
Preface
Before you begin to use the Protocol document, it is important to have a basic understanding of the context of its use and why it is important.
It is also helpful for you to understand the trigger or circumstance that initiates the need to use the functions of the Protocol document, and the fundamentals of its life cycle – when it is required, who completes it, its routing, etc.
Business Needs and Purpose
The animal protocol review and approval process is required by the Animal Welfare Act, the regulations of the United States Department of Agriculture (USDA), and Public Health Service to be in compliance with the Animal in Research Act (99-158) as well as regulations of other federal, state, and granting agencies.
To be in compliance with animal use regulations, all educational and research organizations must constitute an IACUC that has the authority over the use of vertebrate animals in their facilities and over all use activities conducted by their faculty, students, and employees. The IACUC must review and approve all (1) research, (2) education, and (3) use [collectively called ‘use’] before the proposed activity can be initiated.
The Protocol document is completed whenever:
• An investigator wants to submit a protocol application to their IACUC
• An investigator needs to revise, amend or renew their protocol
• An investigator needs to notify the IACUC of an issue or request a change to the status of the protocol
The Protocol document is used to:
• Ensure compliance with institutional research requirements
IACUC Protocol Review
According to PHS policy there are two valid methods of IACUC review
a. Full committee review by a convened quorum of the members of the IACUC
b. Designated member review by one or more members
Designated member review can only be allowed after all voting members have had the opportunity to call for a full-committee review. The specific method of review for a given protocol must be documented within the system along with the outcome of the review determination process.
If any member requests a full committee review, then the full committee review method must be used. If a determination is made to go through the designated member review process, either the IACUC administrator or the IACUC office must be able appoint one or more qualified IACUC members to serve as the designated reviewers. The designated members must be unanimous in any decision. They must all review identical versions of the protocol and if changes to the IACUC protocol are requested by any one of the reviewers, then the other reviewers must be aware of and agree to the modifications. A designated member review process may result in approval, a request for changes to the IACUC protocol (sent to the researchers) and referral to the full committee for review. A designated review may not result in withholding of the approval.
Permitted Actions by Role
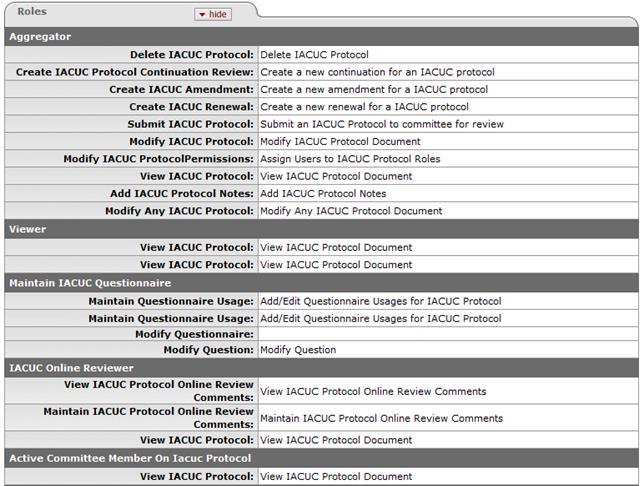
Figure 814 Protocol Document Permissions
|
|
For more detailed information about assigned user roles, see “Permissions” on page Error! Bookmark not defined.. |
Figure 380 Simplified Protocol Document Workflow - Process Flow Chart Diagram
|
|
|
Access
Access to the Protocol document is based on Groups, Roles, Permissions and Responsibilities that are defined in the Kuali Identity Management (KIM) module. Generally speaking, the KC user interface allows investigators to create new Protocol documents, while Unit and Central Admin users are able to search for and edit them.
|
|
For more functional information about e-doc access, see “Access” on page Error! Bookmark not defined. in Overview. |
|
|
Table 417 Protocol Document - Key Concepts
|
Concept |
Description |
|
Available Actions |
These change dynamically based on role and status. |
|
Online Review |
This is an additional page that appears on the far right of the other page tabs when applicable. |
|
Other Documents |
The Protocol document works closely with the Committee document to accomplish IACUC approval. Additionally, it relies on complex maintenance documents such as Questionnaire for customization. |
Creating a new Protocol document
A Protocol e-doc may only be created by an authorized person with the Create Protocol role in KC. This is done by clicking the Create IACUC Protocol link in the Actions section of the IACUC Protocols group on the investigator menu.
 >
>
 >
>  >
> 
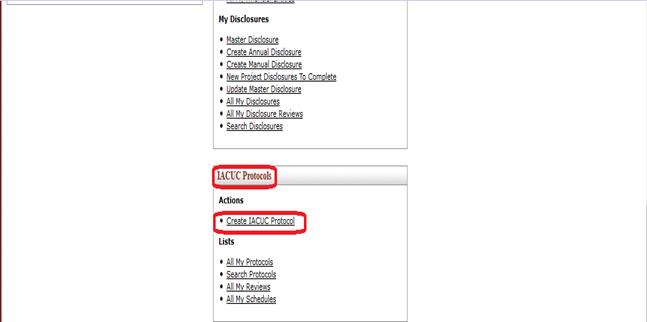
Figure 816 Create Protocol from the Investigator menu
|
|
For more information about creating a new e-doc, see “Initiating a Document” on page Error! Bookmark not defined. in Common E-Doc Operations. |
Accessing an existing Protocol document
To work on an existing Protocol document, an investigator may simply click the Search Protocols link from the Lists section of the Protocols group.
 >
>
 >
>  >
> 
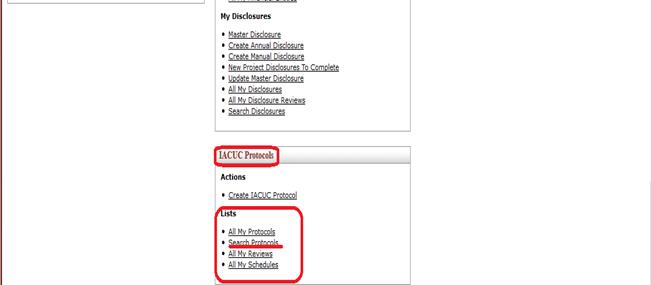
Figure 817 Search Protocols from the Investigator menu
From the Unit or Central Admin menus, you may search for an existing, submitted Protocol document by using the IACUC Submissions lookup in the Post-Submission Compliance group.
 >
>
 >
>  >
>  >
>  >
>  >
> 
An existing Protocol document may also be accessed via the action list or doc search global buttons at the top, left of any KC screen.
|
|
For more information about using the action list, see “Using the Action List” on page Error! Bookmark not defined. in Common E-Doc Operations. |
|
|
For more information about using the doc search, see “Searching for a Document” on page Error! Bookmark not defined. in Common E-Doc Operations. |
Navigation Path Quick Start: Getting started – this quick system reference depicts how to access the start screen for this e-doc.
Create New:
 >
> 
|
|
For more information on creating a new document, see “Initiating a Document” on page 107 in Common E-Doc Procedures. |
Access Existing:
 >
> >enter/select>
>enter/select> >
>
Or…
 >
> >
>
Or…
 >
> >
>
 >
> 
Or…
 >
>
 >
>
 >
> >
>
|
|
For more information on accessing an existing document using the global doc search button, see “ Searching for a Document” on page 114 and “ Doc Search” on page 78 in Overview.
| |
|
|
For more information on accessing an existing document using the global action list button, see “ Action List” on page 72.
|
|
Document Layout
The Protocol document is comprised of a header area at the top, right; 13-14 tabbed pages (Protocol, Personnel, The Three R’s, Questionnaire, Species/Groups, Procedures, Protocol Exception, Custom Data, Special Review, Permissions, Notes & Attachments, IACUC Protocol Actions, Online Review, and Medusa) organized horizontally across the top; a page body area that dynamically displays content based on the currently-accessed page; tabbed, expandable/collapsible sections containing fields on each page; and an action buttons area at the bottom, center of any page.
Table 418 Protocol Document Major Components Overview
|
Major Document Component |
Summarized Description |
|
Header area |
Document identification information at the top, right of the document containing both common and document-specific fields. |
|
Tabbed Pages |
Pages that make up the document which are accessible by clicking the folder tab for each. These are groupings of functionally related information for the purpose of display and collection and are generally designed to be completed in left-to-right order. |
|
Tabbed Page Sections |
Multiple tabbed sections of each page containing data entry/selection/display fields that are expandable/collapsible via hide/show buttons. Some sections contain subsections – the labels for which are highlighted in a darker shade of grey. |
|
Action Buttons |
Buttons that appear at the bottom, center of the document (regardless of page), some of which are common to all e-docs, and some of which are unique to this particular document. |
|
|
For more information about e-doc component features that are common to all e-docs, see “E-Doc Topology” on page Error! Bookmark not defined. in Overview > E-Doc Fundamentals. |
|
|
For more information about e-doc component features that are unique to the Protocol e-doc, scroll or click to navigate to the subsequent subtopic headings for each page and section that follows below. |
Document Header
The document header area of the Protocol document is comprised of six display-only fields at the top, right of the document. This is viewable regardless of currently-accessed page, and contains a minimum amount of basic information about the Protocol document to allow you to be sure you have accessed the correct document. Some fields are populated upon creation, while others display information only after subsequent user action. Each is described in the table that follows.

Figure 818 Protocol Document Header Example
Table 419 Protocol Document Header Field Descriptions
|
Field |
Description |
|
Document Id |
The system-generated number that uniquely identifies the currently-displayed Protocol document, this is assigned upon initiation (create action from Investigator menu). |
|
Initiator: Last Updated |
Displayed when a new protocol is created, this is the User ID of the logged-in user who last updated the Protocol document and the timestamp of the update. This also serves as a link to additional information about the user. |
|
Protocol # |
After you populate the required fields on the Protocol page and click the save button, the Protocol # is automatically assigned by the system. |
|
Status |
This is the protocol status and describes the state the Protocol document is in, which differs from its workflow routing status. |
|
Submission Status |
The submission status provides additional information of the protocols state after it has been submitted to the IACUC. |
|
Last Updated |
The time and date is displayed after the first save action, and updated with each save thereafter. |
|
Expiration Date |
Generated by an approval action, this is the last day of the current approval period, and changes due to a renewal approval action. |
Pages Overview
The Protocol document is made up of 13-14 pages, each of which is accessed by clicking its corresponding tab. Upon initiation, the default view is the Protocol page. Each page is labeled according to the type of information collected, and contains tabbed, expandable/collapsible sections containing fields with entry and selection tools.
|
|
Page Order & Completion: The pages in KC e-docs are designed with the intent that you complete them in left-to-right order, although this is not required. In general, you may choose to work on any page in any order to complete the document. The one caveat is that in order to proceed to any page from the Protocol page, you must first fill out the required fields on the Protocol page and then click save. |

Figure 819 Protocol Document – Horizontal Page Tabs Layout
Table 420 Protocol Document - Topic Links & Page Descriptions
|
Page Name & Topic Link |
Description |
|
Protocol |
General information about the Protocol |
|
The people involved in doing the work for the research project | |
|
The Three R’s |
Principles of reduction, refinement, replacement, and alternate searches performed |
|
The protocol questions themselves and selection/entry tools for answering them | |
|
Species/Groups |
Species of animals being used and experimental groups |
|
Procedures |
Overview and timeline for all procedures and details for each individual procedure. |
|
Protocol Exception |
Deviation or exceptions from standard procedures |
|
Institution-specific data you’d like to record | |
|
Add specialized review types and track related status & dates | |
|
Assign roles to users (who can do what with regard to completion of the protocol) | |
|
Add textual comments or documents related to the protocol or personnel information | |
|
Perform tasks via commands such as submit, notify, expire, validate, print, copy, route, etc. | |
|
Medusa |
Allows viewing of e-docs associated with the protocol with a hierarchical tree view. |
Action Buttons
Standard action buttons appear at the bottom, center of the screen, regardless of currently-accessed page, as is true with any e-doc in KC.

Additional action buttons appear according to:
• the currently-accessed page
• the logged-in user’s permissions
• the document passing through various stages of completion
• the document passing through various stages of approval routing

|
|
For more information about action buttons, see “Action Buttons” on page 71 in Selection, Entry & Action Tools. |


 Protocol
Protocol