Special Review
The Special Review page has a single tabbed section also
labeled Special Review that provides the standard add line item functionality
described in the Common E-Doc Operations section of KC Overview.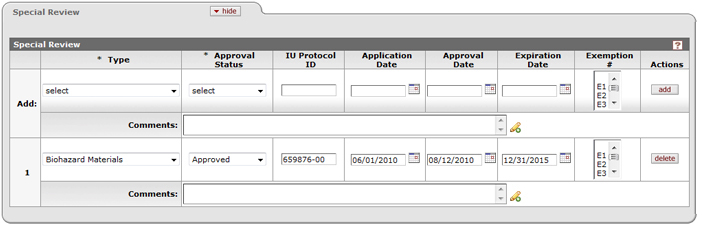
Figure 183 Proposal Development Document > Special Review Page > Special Review Section – Example
Table 77 Proposal Development Document > Special Review Page > Special Review Section – Field Descriptions
|
Field |
Description |
|
Add |
The line where you make your entries and selections and click the add button, which adds them as numbered line items below. |
|
Type |
Required. The type indicates which special
review, if any, is relevant to the document (proposal/award/irb).
Type selections include use of animal usage, biohazard materials,
foundation relations, human participants, international relations,
radioactive isotopes, recombinant DNA, space changes, TLO PR-Previously
Reviewed, TLO Review – no conflict (A), TLO Review – potential conflict
(B2), TLO Reviewed – no conflict (B1).Click the down arrow |
|
Approval Status |
|
|
IU Protocol ID |
The unique identifier assigned to the protocol
submitted for regulatory review. The protocol # is the number assigned to
the protocol submitted by the PI to the institution's relevant committee
(e.g. IACUC, IRB, etc.). A unique number assigned by the institution
and/or compliance committee to a specific research Protocol. Type the
value if known in the box, otherwise, search for and select it and
populate the box automatically by using either the lookup |
|
Application Date |
The date the protocol was submitted to the
regulatory committee. The application date is the date the PI submitted a
protocol/request for permission to the relevant institutional
committee/person for special Review. Enter the date in mm/dd/yyyy format or select it
using the date selector by clicking on the calendar icon |
|
Approval Date |
The date the protocol was approved by the
regulatory committee. The approval date is the date the protocol/request
for permission was approved by the relevant institutional
committee/person. Enter the date in mm/dd/yyyy format or select it
using the date selector by clicking on the calendar icon |
|
Expiration Date |
The date, after which, the existing protocol expires.
Enter the date in mm/dd/yyyy format or select it from the calendar
selector to specify the date this special review will expire. Enter
the date in mm/dd/yyyy
format or select it using the date selector by clicking on the calendar icon |
|
Exemption # |
One of six categories of exempt research specified by the Common Rule (45 CFR 46.101(b). If the proposed project is exempt, as defined by the different institutional committees, an exemption number is generally issued, and is entered into this field. Scroll the spin box to view the list items from which to select. |
|
Comments |
Type to enter internal notes relevant to the protocol or special review item. |
|
Actions |
After line items have been added, they may be removed by clicking the delete button. |
Linking To A Protocol Document
The Special Review pag e is integrated with the Institutional Review Board module to allow the linking of Proposal Development and Institutional Proposal documents to Protocol and Committee documents. This also works if Institutional Proposal is then linked to an Award document. The ‘irb.protocol’ parameter enables the linking of Protocol to Proposal and Award.
|
|
To link to a Protocol document: |
|
1. |
In the Type field,
select ‘Human
Subjects’ from the list. Use
the drop-down The Protocol Number column field becomes editable with a lookup icon. |
|
2. |
Enter the document
number for the Protocol document you want to link to, or click the
lookup The Approval Status, Application Date, Approval Date, Expiration Date and Exemption # fields are automatically populated based on your selection when the data exists. |
|
3. |
Enter comments as
desired. Click within the text box (or press the tab |
|
4. |
Click the add
Your selection/entry is added as a numbered line item row below the Add row. Viewing The Linked Document |
|
5. |
Click the view
The Protocol document appears in a new browser window. |
|
End of activity. | |
 to display the list and
click on an item in the list to highlight and select it to populate the
box with your selection.
to display the list and
click on an item in the list to highlight and select it to populate the
box with your selection.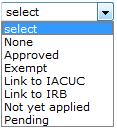 Required. Select from the list to
indicate if the special review relevant to the document
(proposal/award/irb) is pending approval, or already approved.
Required. Select from the list to
indicate if the special review relevant to the document
(proposal/award/irb) is pending approval, or already approved. or
or  functions.
functions. .
.

 key from a previous field) to
reposition the cursor so that it is within the field, and then type (or
paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the expand text
key from a previous field) to
reposition the cursor so that it is within the field, and then type (or
paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the expand text
 icon to display a
pop-up window with an expanded text entry area if you want more screen
real estate to type in, and then click the continue button to close
the window and return. After text has been entered and saved, click
the green arrow
icon to display a
pop-up window with an expanded text entry area if you want more screen
real estate to type in, and then click the continue button to close
the window and return. After text has been entered and saved, click
the green arrow icon to read it in its entirety in the larger pop-up window, and then
click the close button to close the window and
return.
icon to read it in its entirety in the larger pop-up window, and then
click the close button to close the window and
return. button in the
Actions column.
button in the
Actions column. button in the
Actions column for the numbered line item row of the document you want to
view.
button in the
Actions column for the numbered line item row of the document you want to
view.