Other Identifiers
The Other Identifiers subsection of the Additional Information section on the Protocol page gives you the ability to track identifiers (other protocol numbers or names) used by other study sites or sponsors. Select from a list of identifier types (for example, Radiation Therapy Oncology Group), and add corresponding identification values as necessary for your tracking requirements.
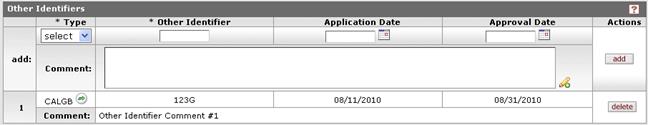
Figure 637 Protocol Document, Protocol Page, Additional Information Section, Other Identifiers Subsection Example
Table 273 Protocol Document, Protocol Page, Additional Information Section, Other Identifiers Subsection Column Descriptions
|
Column |
Description | ||
|
add / # / Comment row |
Make your entries/selections from the add row, then click the add button in the Actions column to add the information as a numbered line item.
To remove a line item, click the delete button in the Actions column for the corresponding numbered row.
To add comments, click
within the text box (or press the tab | ||
|
Type |
Required. To specify an identifier type, use the
drop-down CALGB: Cancer and Leukemia Group B RTOG: Radiation Therapy Oncology Group IRBNet: online research administration tools site COAG: Clarification of Optimal Anticoagulation Through Genetics research network
| ||
|
Other Identifier |
Required. To enter the short text field code (at
least one character) that uniquely identifies and represents the line item
you will be adding, click within the text box (or press the tab
| ||
|
Application Date |
Click the calendar
| ||
|
Approval Date |
Click the calendar | ||
|
Actions |
Make your entries/selections from the add row, then click the add button in the Actions column to add the information as a numbered line item.
To remove a line item, click the delete button in the Actions column for the corresponding numbered row.
|


 key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information.
key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information.


