Additional Information
The Additional Information section of the Protocol page collects FDA-related information.
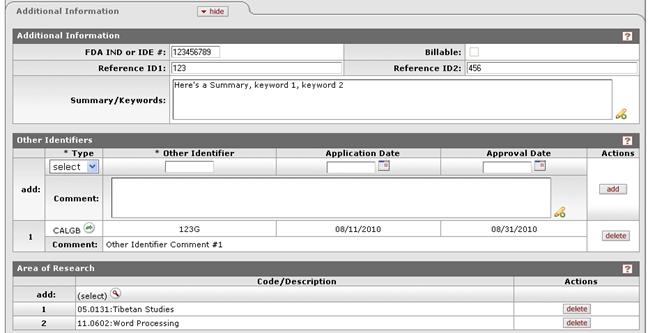
Figure 635 Protocol Document, Protocol Page, Additional Information Section – All Subsections Example
Table 272 Protocol Document, Protocol Page, Additional Information Section Field Descriptions
|
Field |
Description |
|
FDA IND or IDE # |
Enter the Investigational New Drug (IND) or Investigational Device Exemption (IDE) number, when applicable. |
|
IU or Investigator held IND/IDE? Y/N |
Type either “Y” or “N” to indicate if the IND or IDE is held by Indiana University or an IU investigator. |
|
Summary/Keywords |
Optional. Click within the text box (or press the tab key from a previous field) to type (or paste from virtual clipboard) text as necessary to provide a brief summary of the research. Click the add note icon to view/edit/paste text in a larger browser window, then click the continue button to return to the text entry field in the document. |
|
Reference ID2 DO NOT USE |
For protocols that existed prior to the implementation of KC IRB, this field identifies the Grant or Sponsor # associated with the protocol. This information will eventually be removed and housed solely in the Funding Sources panel. |