Institutional Fields Conditionally Required
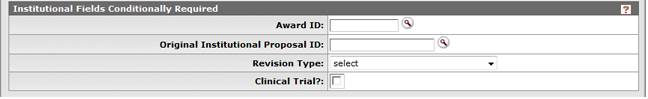
Figure 148Proposal Development Document, Proposal Page, Required Fields for Saving Document Section, Institutional Fields Conditionally Required Subsection Example
Table 56Proposal Development Document, Proposal Page, Required Fields for Saving Document Section, Institutional Fields Conditionally Required Subsection Field Descriptions
|
Field |
Description |
|
|
A unique identifier assigned
by an institution to identify an award (e.g. used when submitting a
continuing type of activity.). A unique institutionally assigned
number of a previously funded application. A unique ID automatically
assigned by the system (based on institution specifications) to identify
an award. An award is defined as: To grant or declare as
merited or due. If, after consideration, an outside agency/sponsor decides a proposal has merit and
worth, an agreement is drawn between the funding agency and Institution
for services. This agreement is called an award. An award is a legal,
binding document for services in lieu of funds to perform the original
outline of the proposal submitted. Click the lookup |
|
Original Institutional Proposal ID |
The unique identifier
originally assigned to the previously-submitted proposal record;
used if the document is being revised and/or resubmitted. The
number assigned by KC
Proposal Development module for the previously submitted proposal record
currently being revised. A unique institutionally assigned proposal
number of a previously submitted proposal. This is applicable when
submitting a revised (changed/corrected) application. Click the
lookup |
|
Revision Type |
This drop-down menu provides a number of options related to the proposal development document. |
|
Clinincal Trial (Check box) |
Check this box if a Clinical Trial is being conducted. This queries the KC system to include the Clinical Trials Office in the routing chain. |
